Current and Emerging Treatments
Current Pharmacologic Approaches to the Treatment of Parkinson’s disease (PD)
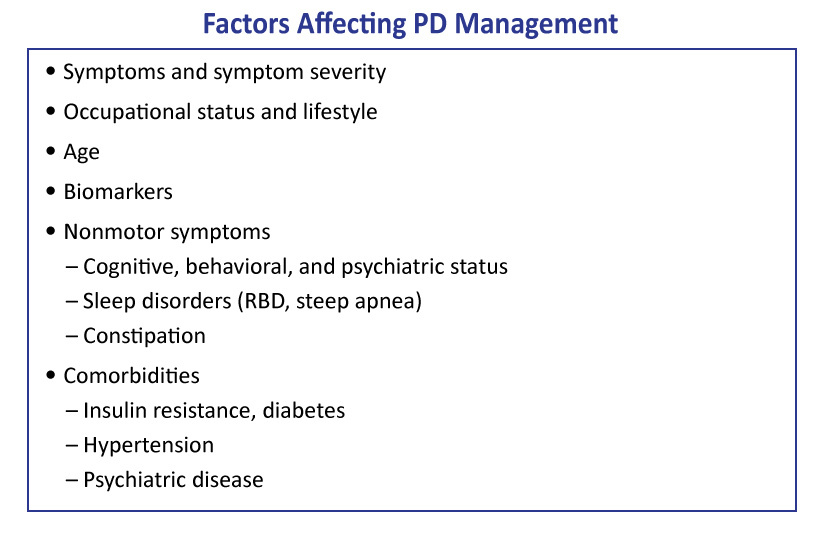
When selecting the appropriate treatment regimen for a patient with PD, a highly individualized approach is recommended.1 The patient’s age; symptoms; symptom severity; occupational status; lifestyle; cognitive, behavioral, and psychiatric status; and other medical comorbidities should all be among the many factors to be considered.(Figure 1)
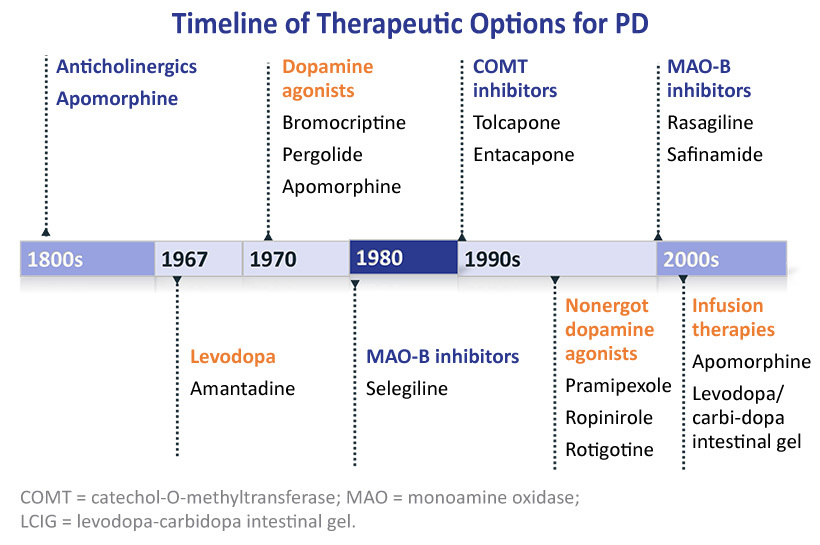
All currently approved pharmacotherapies for treating PD aim to increase striatal dopamine levels to ameliorate associated motor deficits.(Figure 2)2 Unfortunately, these approaches do not represent a long-term solution, as each loses efficacy as dopaminergic neurodegeneration progresses over time.
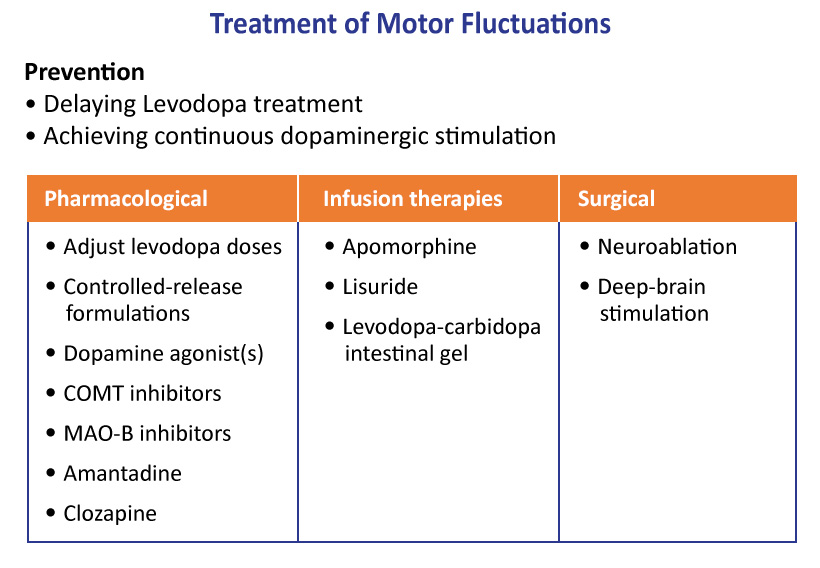
Strategies for managing motor fluctuations focus on pharmacological (oral/infused/device-aided) and surgical options. (Figure 3)
Levodopa and Carbidopa
Levodopa (L-DOPA), which is biosynthetically transformed to dopamine via aromatic L-amino acid decarboxylase, is commonly given to augment dopamine levels in PD patients. Since its introduction in 1967, L-DOPA has persisted as the gold standard for treating PD symptoms and is an integral component of combination therapies. L-DOPA tends to lose efficacy over time, with greater than 80% of patients on therapy for longer than 10 years experiencing dyskinesia and ‘‘on-off” periods.3 The ‘‘on-off” motor fluctuations consist of an initial benefit after a dose of L-DOPA (on-time) followed by a return of parkinsonian features (off-time) before onset of benefit from the subsequent dose.4 Carbidopa is often given in combination to reduce systemic metabolism of L-DOPA and increase central exposure, allowing lower doses of L-DOPA to maintain efficacy while reducing side effects such as nausea.2
Continuous dopaminergic stimulation is a strategy aimed at decreasing motor fluctuations in advancing PD. Three device-aided treatments are currently available that provide different kinds of continuous stimulation: Levodopa/carbidopa intestinal gel infusion, deep brain stimulation (DBS), and subcutaneous apomorphine pump infusion (not approved in U.S.). The intestinal gel, for example, continuously delivers L-DOPA via percutaneous endoscopic gastrojejunostomy (PEG-J), and is used in PD patients who experience motor fluctuations not adequately controlled by oral dopaminergic medications.
Dopamine Agonists
Dopamine agonists act on dopamine receptors to amplify the effects of dopamine. Approved therapies that agonize dopamine receptors and are prescribed for treating PD include apomorphine, bromocriptine, ropinirole, pramipexole, and rotigotine.5 Each of these molecules is most potent as an agonist of D2-like receptors with several also possessing some balance of D1-like agonism and 5-HT, α-adrenergic and β-adrenergic antagonism. Apomorphine sublingual film is a recently-approved dopamine agonist. It is a fast-acting sublingual film for the on-demand management of off episodes associated with PD. The sublingual film formulation is designed to offer a potential solution that may be used to treat motor off episodes associated with PD up to five times throughout the day, which may help people with PD rapidly convert from the off to the on state.
MAO-B Inhibitors
Monoamine oxidase B (MAO-B) is an enzyme involved in the metabolism of dopamine forming 3,4-dihydroxyphenylacetic acid which is eventually transformed (by catechol-O-methyltransferase (COMT)) into homovanillic acid (HVA).6 MAO-B inhibitors have been incorporated into the therapeutic regimen for PD treatment to decrease dopamine metabolism and thereby increase dopamine concentrations in the brain, leading to a reduction in motor symptoms.2 Approved MAO-B inhibitors include the irreversible inhibitors selegiline and rasagiline, as well as the reversible inhibitor safinamide.
COMT Inhibitors
COMT is an enzyme that catalyzes a parallel metabolic pathway transforming dopamine into 3-methoxytyramine, which is subsequently oxidized by MAO-B to produce HVA. Similar to MAO-B, inhibition of COMT leads to an increase of brain dopamine levels, reducing motor symptoms, and has been added to PD treatment over the past two decades as a combination therapy.2 Entacapone and tolcapone are approved COMT-inhibitors now used as components of standard of care.
Figure 4: Current Treatment Algorithm for PD
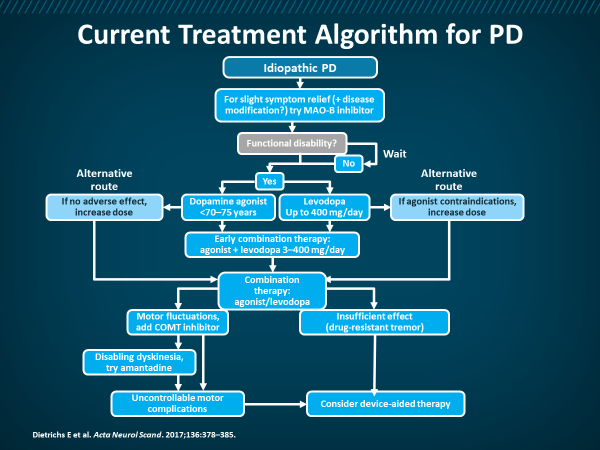
Other Agents/Targets
Anticholinergics do not act directly on the dopaminergic system, but instead modulate the activity of acetylcholine, which is involved in regulating movement, and can have beneficial impacts on tremor and dystonia in PD patients.2 Benzatropine and trihexyphenidyl are two approved antiparkinsonian therapies that decrease the activity of acetylcholine.
Several alternative mechanisms are employed in managing symptomatic PD manifestations.2 Amantadine is an N-methyl-D-aspartate-type glutamate receptor antagonist historically used as an antiviral therapy. Droxidopa, a prodrug of norepinephrine, was recently approved in the U.S. for managing neurogenic orthostatic hypotension in disorders such as PD, multiple system atrophy (considered a Parkinsonian condition), and pure autonomic failure. For treating hallucinations, delusions, and psychosis associated with PD, pimavanserin, a 5-HT inverse agonist, has been prescribed. Additionally, rivastigmine, an acetylcholinesterase inhibitor, is used to treat PD dementia.
References
- Krüger R, Hilker R, Winkler C, et al. Advanced stages of PD: Interventional therapies and related patient-centered care. J Neural Transm (Vienna). 2016;123:31-43.
- Ellis JM, Fell MJ. Current approaches to the treatment of Parkinson’s disease. Bioorg Med Chem Lett. 2017;27:4247-4255.
- Hickey P, Stacy M. Available and emerging treatments for Parkinson’s disease: a review. Drug Des Devel Ther. 2011;5:241-254.
- Olanow CW, Kieburtz K, Odin P, et al. Continuous intrajejunal infusion of levodopa-carbidopa intestinal gel for patients with advanced Parkinson’s disease: A randomised, controlled, double-blind, double-dummy study. Lancet Neurol. 2014;13:141-149.
- Choi J, Horner KA. Dopamine agonists. StatPearls. Last update June 26, 2023. https://www.ncbi.nlm.nih.gov/books/NBK551686/
- Edmondson DE, Binda C, Mattevi A. Structural insights into the mechanism of amine oxidation by monoamine oxidases A and B. Arch Biochem Biophys. 2007;464:269-276.





